Newsroom
From the Brain to Fingertips: Unlocking Stem Cell Potential for Tissue Regeneration
Writing by Ashleigh Willis from the Miller Lab, Michael Smith Laboratories
The regeneration of human tissues might sound like an idea borrowed from science fiction. Yet, research from Dr. Freda Miller’s lab (Michael Smith Laboratories, Department of Medical Genetics) is revealing that some form of tissue regeneration may be possible. Understanding the mechanisms of regeneration could have a positive impact on human health by helping to repair damage caused by injury or disease.
The key to this potential regeneration lies in stem cells, with researchers in the Miller lab looking at those found in mammals. These stem cells have the potential to turn into many other cell types, and are found in several places throughout the body.
Stem cells arise during development, and are responsible for producing the various cell types that build different tissues. They persist into adulthood where they remain important for the processes like blood and immune cell production and wound healing. However, adult stem cells are less plentiful and less active compared to their counterparts during embryonic life, infancy and childhood. The less active state of adult stem cells is referred to as ‘dormancy’. Despite being less active, dormant adult stem cells do sense their environment and can be activated to generate other cell types from different signals.

Willis setting up an instrument to analyze the spatial location of genes in brain tissue.
Two postdoctoral fellows in the Miller lab, Drs. Ashleigh Willis and Christine Eisner, are investigating whether these stem cells can be mobilized (or, ‘woken up’) by molecules in their environment to repair tissue damage in two distant places: the brain, and the tip of the finger (or, digit).
Waking up stem cells for brain repair
Willis works on stem cells that live in the brain. Previous research suggests that these stem cells decide which type of brain cell to make based on cues from the surrounding environment.Willis’ work asks whether we can ‘wake up’ stem cells by manipulating their environment to treat damage we see in conditions like Multiple Sclerosis (MS).
Neurons in the brain and spinal cord communicate via long fibers. A substance called myelin enwraps these fibers, and enables faster communication. In MS patients, myelin becomes damaged, and the cells which produce and repair myelin, called oligodendrocytes, are also lost. When damaged myelin can’t be repaired, cell communication is interrupted and can eventually stop all together. This results in the symptoms of MS.
So, how can we fix this myelin damage?
“We might be able to repair myelin by recruiting stem cells which are already present in the brain. If we can activate these stem cells and guide them to replace lost oligodendrocytes, then they could repair myelin,” says Willis.
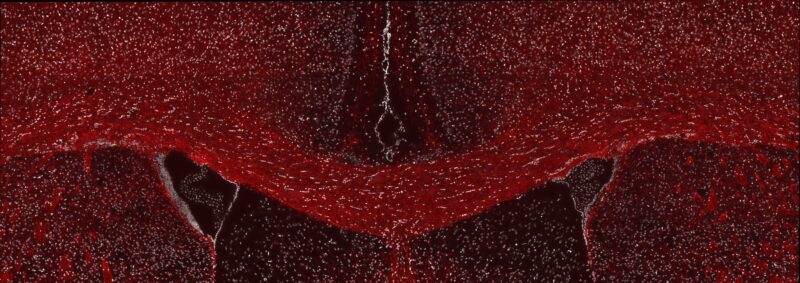
A fluorescent microscope image from Willis’ research showing healthy myelin in the adult mouse brain. The brain section has been labeled for a myelin protein (red) and cell nuclei (white).
Willis’ recent work has used a mouse model of brain injury and repair to uncover two molecules that are much more abundant in the stem cell environment while myelin is being repaired. Her recent experiments have shown that these molecules can activate stem cells to generate more oligodendrocytes when they are added to the surrounding environment..
The substantial effect these molecules can have on stem cell behaviour is an important discovery and, with more investigation, these molecules may represent a therapeutic avenue for myelin repair..
“Finding new treatments for MS is important to me. The two places I call home, Canada and Scotland, have the highest rates of MS in the world. Therapies that could give even a moderate amount of repair would make a huge difference for so many people,” shares Willis.
Regeneration at your fingertips
Eisner’s research is focused on how mammals can regenerate something as complex as the fingertip (or, digit tip, in mice) using stem cells.
“I’ve always been interested in how we can harness the body’s own stem cells to regenerate tissues, but not all tissues regenerate equally. The digit tip is such a unique and complex example of true multi-tissue regeneration, and I believe that if we can better understand the process, perhaps we could apply it to other tissues to enhance regeneration,” emphasizes Eisner.

Eisner at her lab bench pipetting a buffer solution for her immunofluorescent labeling experiment.
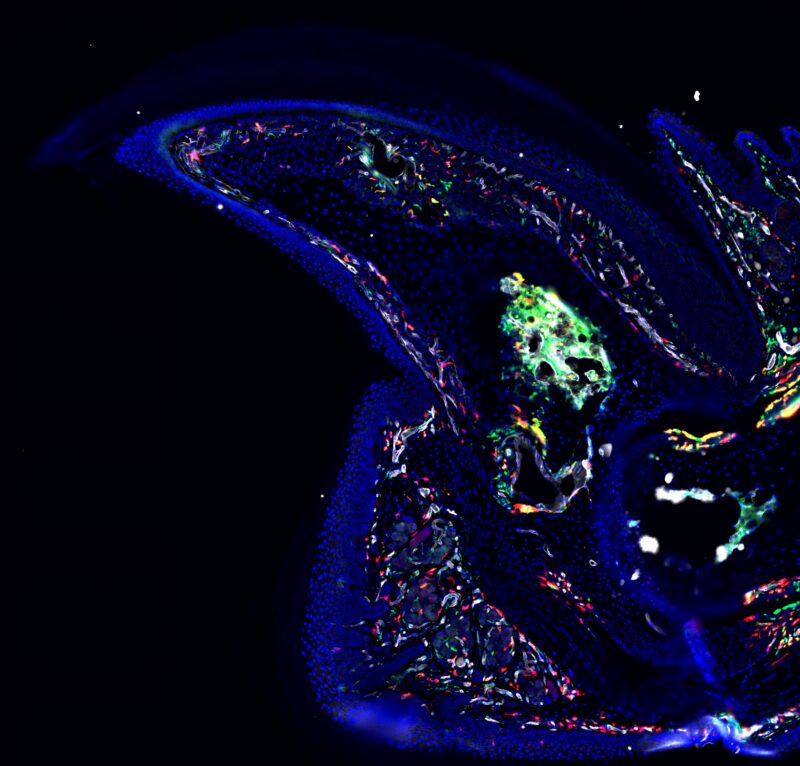
A fluorescent microscope image of the mouse digit tip from Eisner’s research.
Regeneration of complex tissue types isn’t common in mammals, but it does happen in some instances. For example, the tip of the finger in humans (and tip of the digit in mice), if cut off, will regenerate as long as the nail bed is intact. In this remarkable process, we see that the regrown digit looks identical to that before injury. If we can understand how the digit regrows, then we could apply those lessons to promoting regeneration and repair of other injured tissues.
In 2022, Eisner and colleagues in the Miller lab published an exciting breakthrough which showed, in part, why the presence of the nail bed is important to whether the digit will regenerate or scar. Their study identified a subset of nail bed mesenchymal cells, a type of cell that can acquire stem cell-like properties, that are crucial for regeneration. Eisner and the team showed that without these cells, nothing regenerated, and scar tissue formed instead.
Knowing that cells don’t live in isolation, Eisner has since been asking whether the cell environment within the digit might dictate whether or not digit regeneration is possible.
Does the immune system set the stage for regeneration across the body?
Despite working on distant tissues, Eisner and Willis have found similarities in their work: both researchers have uncovered important roles of the immune system during the repair of tissue damage.

Willis (left) and Eisner (right) outside of the Networks of Centres of Excellence building, where Dr. Freda Miller’s lab is based.
The two molecules Willis found to stimulate stem cells for myelin repair were made specifically by brain immune cells called microglia. Her work demonstrates that microglia secrete different molecules into the stem cell environment during myelin repair, and that some of these newly secreted molecules activate stem cells to make myelin-repairing oligodendrocytes. This suggests that they may be used therapeutically to facilitate myelin repair in MS.
Given the role of inflammation and the immune system during injury, Eisner’s focus on cell environments has also led to an investigation of immune cells.
“Immune cell infiltration is a common response after injury, and the digit tip is no exception. I am trying to understand how immune cell population dynamics differ in regenerative and non-regenerative amputations, and how they might influence the regenerative environment.” explains Eisner.
Eisner is now asking how the immune environment differs during regeneration compared to scarring. She is also investigating when the immune environment changes following digit amputation, to understand whether there may be an optimal time for interventions that drive regeneration over scarring. Eisner plans to investigate what molecules immune cells make during these two distinct responses. Her future work will ask whether immune cells and nail bed mesenchymal cells communicate, and whether this communication matters in creating a pro-regenerative environment.
“If we can understand when and how to alter the inflammatory environment after injury to enhance regeneration, it could have a huge impact for patients,” says Eisner.
These two projects highlight that while tissues across the body function differently, there may be similarities in what makes for the right environment for regeneration and repair. If we can better understand these conditions, and repair mechanisms, then we might be able to leverage them to benefit all sorts of injuries and diseases.
Willis’ work is supported by an endMS postdoctoral fellowship from MS Canada. Eisner’s work is supported by CIHR.
Quick links:
- More about Ashleigh’s research on regeneration in the brain
- More about Christine’s research on regeneration of the digit tip
- More about the Miller lab