Newsroom
Hirst Lab decoding the epigenetic cause of muscular deterioration in FSHD
Writing by Fangwu Wang from the Hirst Lab, Michael Smith Laboratories
Living with muscular dystrophy can feel like bearing a weight that gradually grows heavier over time.
Facioscapulohumeral Muscular Dystrophy, or FSHD, is a rare condition affecting approximately 8 in 100,000 individuals. It is a genetic muscle disorder characterized by the progressive weakening and loss of skeletal muscle, particularly in the face, shoulders and upper arms. It starts with subtle signs, like difficulty whistling or throwing a ball, but the slow progression of muscle weakness eventually makes everyday activities harder or even impossible.
Scientists have identified the genetic variation responsible for FSHD, located in a specific region of chromosome 4. The disease can arise through two mechanisms, both linked to the abnormal expression of the DUX4 gene in this region, a gene normally expressed only in embryonic cells.
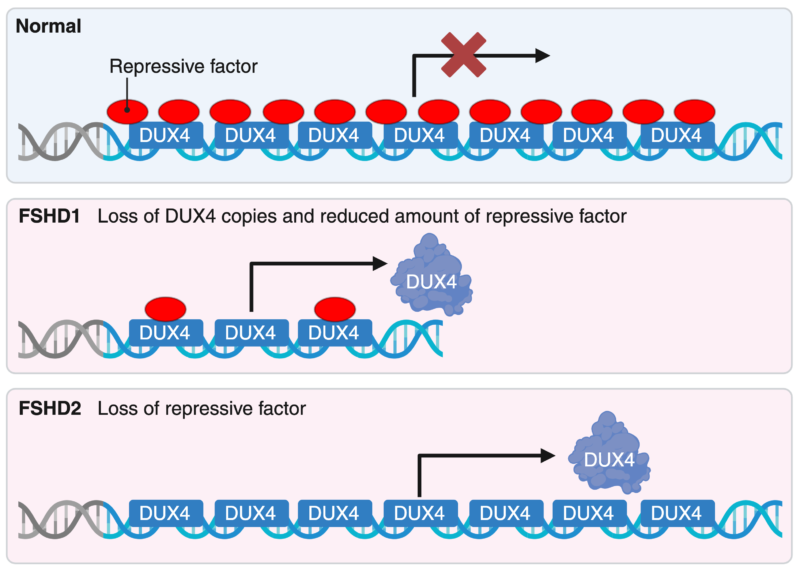
Two mechanisms of pathogenic activation of DUX4 in FSHD. Created with BioRender.com.
There is unfortunately no cure for FSHD largely due to the unique challenges this disease presents: 1) targeting large areas of DNA or silencing the DUX4 gene in muscle cells has not yet been achieved; 2) there is no established biomarker for tracking disease progression in clinical trials; and 3) this disease causes progressive and irreversible damage to muscles, which do not regenerate easily once weakened or lost.
Dr. Martin Hirst (Michael Smith Laboratories, Department of Microbiology and Immunology) and his team of researchers believe that to tackle this disease, we need a better understanding of the fundamental changes that occur in muscle cells, caused by the abnormal activity of DUX4.
Two of the team members, Dr. Joon Lee and Qi (Rachelle) Cao, are involved in a study to decipher the effect of DUX4 on muscle cells using advanced epigenetic techniques. Their study is funded by SOLVE FSHD, a venture philanthropic organization founded by Canadian businessman and philanthropist Mr. Chip Wilson, who himself has been affected by the disease.

Hirst lab member Dr. Joon Lee
Why is the Hirst Lab interested in studying the epigenetic regulation of FSHD?
Epigenetics is the study of inheritable cellular mechanisms, such as chemical modifications on nucleotides and histone proteins associated to the DNA, that can affect gene expression without changing the DNA sequence itself.
Computational biologist Dr. Lee in the Hirst Lab explains that epigenetic regulation is critical in FSHD because it limits the inappropriate activation of the DUX4 gene, which drives the disease. Additionally, the primary function of DUX4 is to regulate gene expression by interacting with specific DNA sequences and other proteins involved in epigenetic mechanisms, which facilitates its role in activating its target genes.
“Epigenetic research can help identify novel therapeutic targets and lead to the development of epigenetic drugs for this disease,” Dr. Lee notes.
How does the lab study epigenetic regulation in FSHD?
Hirst lab member Rachelle Cao
To enable a direct comparison of normal and diseased muscle tissue, the Hirst Lab collaborated with the Zammit Lab at King’s College London. The Zammit lab collected muscle cells from normal and abnormal muscles, from both affected and unaffected patients, and shipped those cells to the Hirst lab. The samples were then processed and made into various epigenomic libraries for high-throughput DNA sequencing by Rachelle Cao.
There are several critical, challenging steps involved in sample preparation for epigenetic library construction. Cells are made permeable, DNA is digested into small fragments, histone proteins are enriched using antibodies, and DNA is purified and amplified. Throughout these processes, it is essential to preserve the intact structure of the epigenome.
Cao, renowned for her ‘magical hands’, has extensive experience creating various types of epigenetic libraries from a wide range of tissue types. During experiments, she observed that certain characteristics of muscle cells, such as their irregular shape and multinucleated structure, might make them vulnerable during certain stages of library preparation.
“When attached cells become detached and suspended, their membrane can rupture and become more permeable,” she explains.
Cao customized the initial steps of preparing the samples, thawing flash frozen dry cell pellets in cold mild buffer solution and then very gently suspending cells to minimize any potential damage to the epigenome. After these modifications, the lab obtained greatly improved epigenomic libraries for the affected muscle samples.
What has been discovered so far?
Dr. Lee led the analysis of data generated from these experiments, which included information on various epigenetic features in muscle cells.
Since DUX4 is responsible for the deterioration of muscle cells, he first investigated whether DUX4 expression affects the activity of genes in muscle cells. His findings reveal that many genes show altered activity when DUX4 is present. Furthermore, he discovered that these changes are dependent on both the amount and duration of DUX4 expression. The effects are markedly different between the high, acute expression of DUX4, such as that induced in the lab, and the chronic, low-level expression observed in patients.
“It is very interesting and exciting to study DUX4 expression in different contexts to understand the progression of the disease,” Dr. Lee says. “What we’ve found suggests that epigenetic changes might accumulate over time, which is consistent with the progressive nature of muscle degeneration in FSHD.”
To explore what regulates the unique gene activities linked to DUX4 expression, Dr. Lee conducted a detailed analysis of the patterns of epigenetic modifications, each playing a specific role in controlling gene expression. By comparing cells with and without the DUX4 protein, he identified specific locations in the genome that are activated by epigenetic modifications only in the presence of the toxic DUX4 protein.
Additionally, these locations are predicted to be regulated by other proteins that guide cellular functions, including myogenic regulatory factors involved in muscle cell differentiation. The precise functions of these regulators, however, are still under investigation.
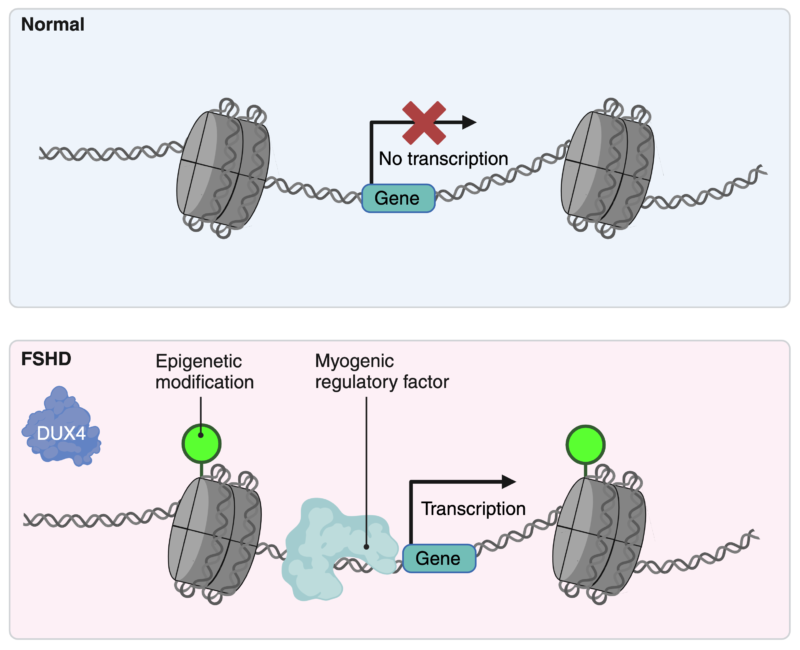
Effect of DUX4 on epigenetic regulation of gene expression. Created with BioRender.com.
Developing a better understanding of epigenetic mechanisms in FSHD offers a promising path toward developing effective treatments for FSHD. The Hirst Lab’s research could lead to better diagnostics and therapies in the future that target the root cause of FSHD, which have the potential to slow or stop muscle weakening and improving patients’ quality of life.