Newsroom
Unlocking the secrets of single cells to improve therapeutics

Leslie Lab members and collaborators (left to right): Albert Kamanzi, Angus Wong, Ella Tate, Robin Coope & Sabrina Leslie
With recent advancements in single-cell analysis and imaging, the MSL’s Leslie Lab is emerging as a leader in this rapidly growing field of research. Working at the intersection of physics and biology, the lab is focused on developing a better understanding of single-cell and molecule interactions that could improve medical therapies.
“The field of single molecule biophysics helps to dissect, understand and apply insights into the interactions and dynamics at the single-molecule and single-cell level to real products. Everything you experience, from the effectiveness of your vaccine to the texture of your sunscreen comes from the interaction of single molecules,” explains Dr. Sabrina Leslie.
In the fall of 2022, the lab received funding in collaboration with the Foster lab for a Pacific Economic Development Agency of Canada (Pacifican) grant totaling $3.5 million with the aim to “Improve the delivery technology behind mRNA vaccines and other nanomedicines”. This enabled them to expand their array of CLiC technology single-molecule and single-cell imaging microscopes. The CLiC platform is an innovative high-resolution microscopy system, translated by UBC-spin-out ScopeSys, used to image single cells and their interactions with molecules, as well as the interactions between the molecules themselves. This “tetherless” technology allows scientists to observe single cells and molecules without the use of additional chemistry that holds them in place, which in turn can affect their behaviours and interactions. In these “life-like” conditions, scientists can observe and measure the properties and interactions of these molecules and apply the information gathered to improve therapeutic design.
How do single-cell imaging platforms work?
Using the CLiC technology platform, researchers flow a solution of cells diluted in a buffer between two sheets of glass. Coated in a special material, cells adhere to the glass and are effectively held in place for observation and imaging. Next, scientists use fluorescent stains to identify molecules of interest. Then these isolated cells are placed into a microscope to observe their properties and interactions when they come in to contact with other molecules, such as drug molecules. This research informs the improvement of drug delivery systems like the lipid nanoparticle technology used with mRNA vaccines, helping to optimize dosages.
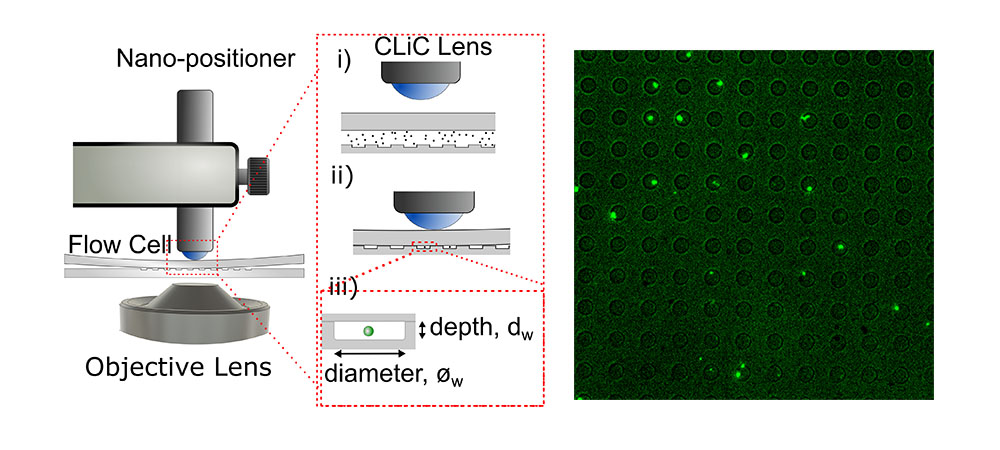
CLiC technology can isolate and hold mRNA-lipid nanoparticles in the field of view for long times.
Why is imaging single cells and molecules so important?
“Life begins with single molecules and single cells, one example being the sperm and the egg. They’re foundational,” reflects Dr. Leslie. “Tools to look at single cells are integral for understanding how biology works at its most fundamental level, but also how it might go wrong and lead to the onset of disease.”
The Leslie Lab’s leadership in this burgeoning field has earned them further funding from a Nanomedicine (NMIN) Phase 4 grant to develop two ongoing projects. The first project aims to scale up an assay (an in vitro procedure used to detect, quantify and study the behaviour and interactions of molecules) which allows scientists to measure the size and mRNA payload for lipid nanoparticles (LNPs) and gain insights into their structure. With a current pipeline for obtaining this information designed on a small scale, this grant allows the lab to team up with engineers and collaborators to scale up the process to high-throughput levels. By compiling these rich, multilayered data sets and correlating these findings with existing data, researchers hope to use this knowledge to create a unique universal platform for broad application.
“Together with others in the nanomedicine community we’re building a general platform which could be used for vaccines, cancer treatment or other personalized medicine applications,” says Dr. Leslie.
Later this year the lab will synthesize their findings on LNP size and mRNA payload, as well as lipid-nanoparticle fusion dynamics, in two upcoming publications.
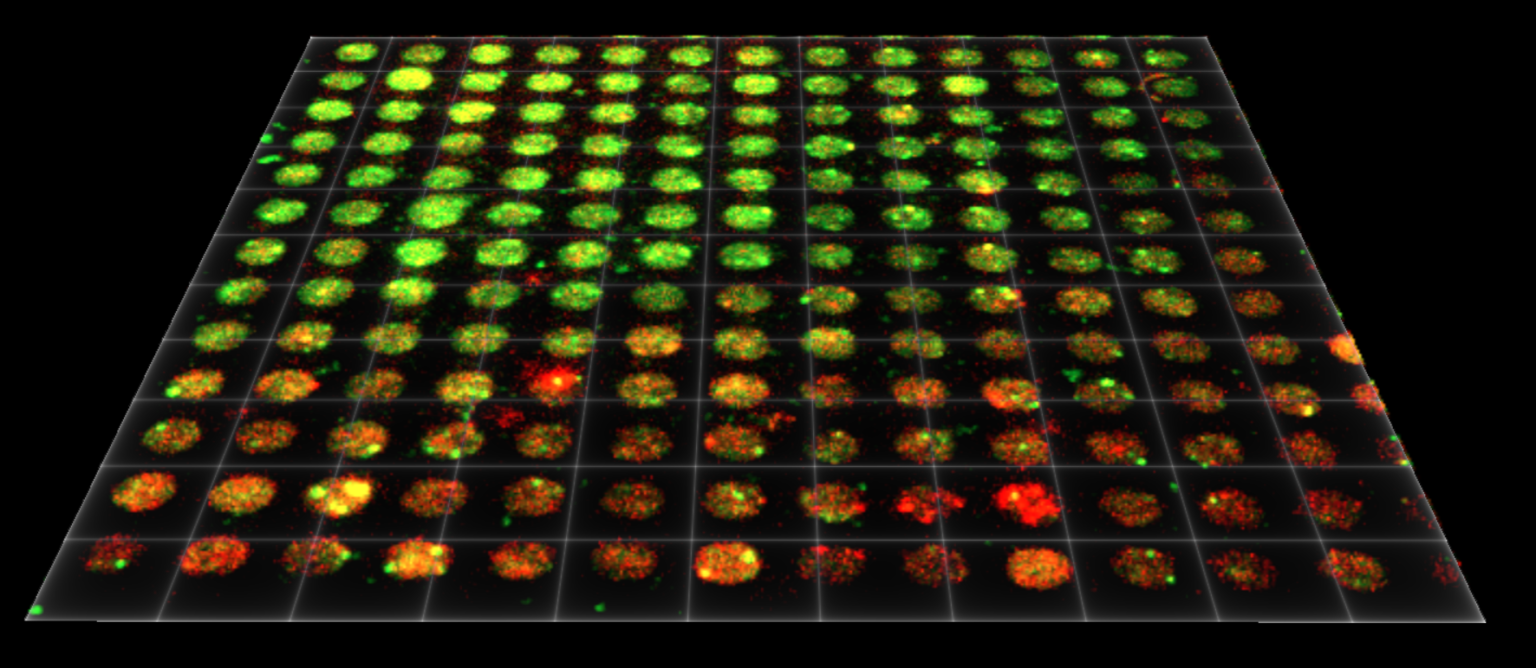
Directly imaging the fusion of mRNA-LNPs in response to change in pH, in real-time
Knowledge to improve vaccines therapies
The second NMIN Phase 4 grant funded project expands on the knowledge gathered with the first assay and explores how these single cells and molecules interact with other particles.
“Our hypothesis as biophysicists is that these fundamental properties matter and they can each be optimized for therapeutic function,” explains Dr. Leslie “The outcome of these studies is a better understanding of how particles fuse, their timescales, and the various parameters the process depends on. This new knowledge will then inform us about the journey of the therapies within cells and improve manufacturing of delivery methods and the therapies themselves.”
The research team is analyzing the interactions of RNA vaccines and therapies with other cells with the hope to improve their development and administration. Current aims for the project include optimizing their potency and extending their shelf life, while reducing the dosages required as well as potential side effects.
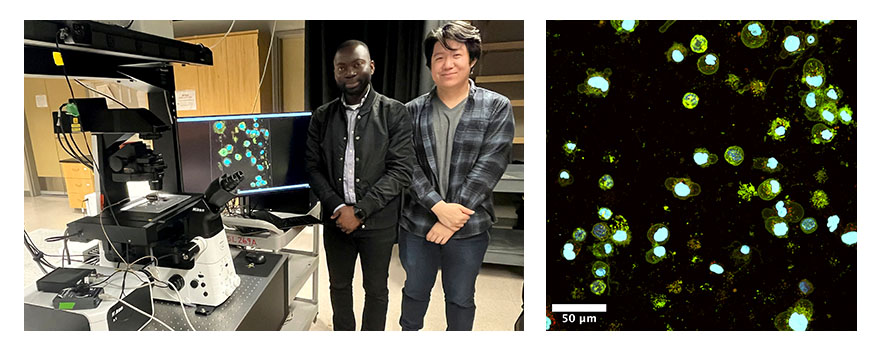
PhD students Eric Boateng and Yao Zhang imaging single cells in the lab
“This second project is a granular look at mRNA-LNP molecules, their properties, their dynamics and the cellular activity. Understanding these mechanisms could help eliminate unlikely candidates while isolating promising ones,” Dr. Leslie says. “We are grateful for support from vaccine and drug developers in the Nanomedicine network, engineers at Genome BC, and industry advisors and the biotechnology community. Their support will help us to translate our findings to real solutions for patients in the future.”
At its essence, the Leslie Lab’s work is about connecting microscopic properties to our macroscopic experience. How such small components shape our world is a fascinating question this research team is unravelling. Importantly, understanding these single cells and molecules at this fundamental level will lead to real world advances in medical therapies, product development and beyond.