Newsroom
Unveiling the Invisible Defenders: How Our Cells Differ in Viral Combat
Writing and photography by Osei Boakye Fordwour from the Foster Lab, Michael Smith Laboratories
In the ongoing quest to understand the complexities of human biology, graduate student researcher Lucy Chi from the laboratory of Professor Leonard Foster (Michael Smith Laboratories, Department of Biochemistry and Molecular Biology) has embarked on a pioneering study that illuminates the intricate ways in which our brain cells, specifically astrocytes, engage in the fight against viral infections using mass spectrometry. Whereas previous research utilized traditional bulk scale analyses, here single-cell proteomics (SCP) was employed to explore how these cellular defences vary between sexes.
Discovering diversity in defence
Astrocytes, the star-shaped cells in our brains, play a critical role in the central nervous system’s immune defence. These cells were traditionally studied in groups, but this approach misses individual cell nuances. The emergence of single-cell proteomics has now enabled a deep dive into cells’ unique viral responses.

Lucy Chi culturing astrocyte cells in a biosafety cabinet.
This innovative approach has helped Chi uncover significant differences in how male and female astrocytes react to infections, such as those caused by the Human Coronavirus 229E (HCoV229E).
“Analyzing the proteomic profiles of these cells has revealed a diverse landscape of cellular defence mechanisms that vary not just between individuals, but also between sexes,” explains Chi.
Taking a closer look at the findings
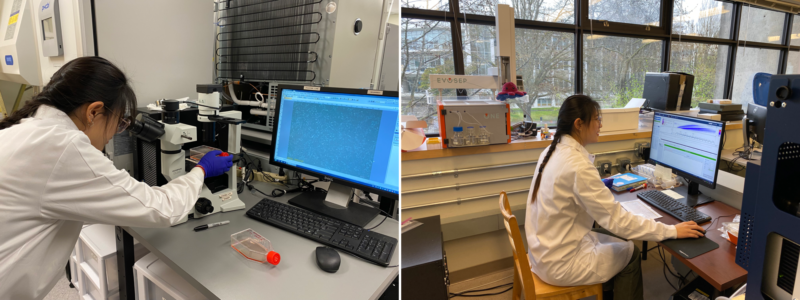
The left image shows Lucy observing primary astrocytes under a microscope. On the right, she is seen analyzing the proteomic profiles of the astrocytes on a mass spectrometer.
The study’s preliminary data is revelatory.
“Through experiments under identical conditions, I identified that male astrocytes have faster growth rates and exhibit shorter branch arm lengths than their female counterparts, which is quite fascinating,” says Chi.
Proteomic analysis brings to light distinct profiles between the sexes with over 300 significant variations identified in bulk studies. This difference in protein expression aligns with observed growth rates and suggests a deeper, molecular-level explanation for sex-based variations in disease response and recovery. Similar analyses of infected astrocytes also display interesting findings, with male astrocytes showing a stronger immune response to HCoV229E infections than those of females.
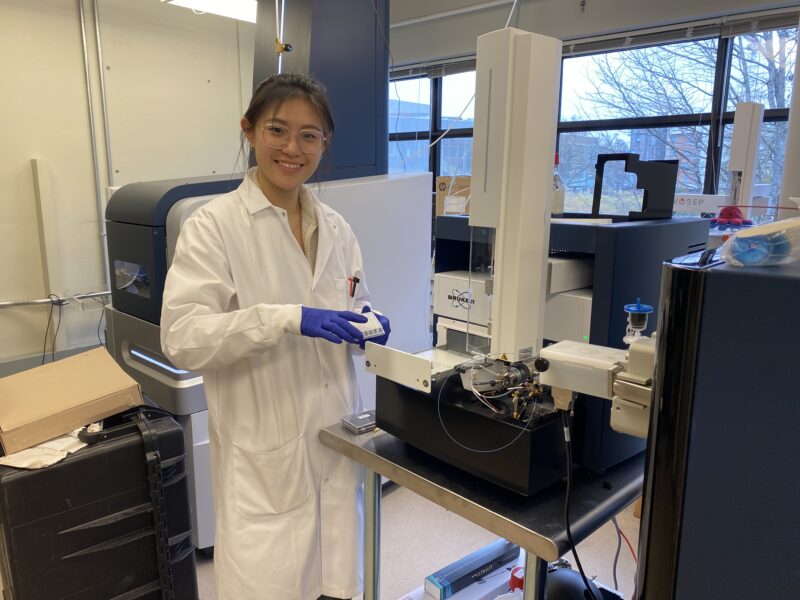
Lucy loads samples onto the SCP mass spectrometer.
Implications and future directions
The implications of these findings are vast, with potential impacts on everything from the development of targeted therapies, to our understanding of disease progression.
As Chi mentions, “with the discovery of the sex differences between bulk female and male astrocytes in mind, utilizing single-cell proteomics will illuminate the distinct roles individual female and male astrocytes play in contributing to disease conditions.”
This research underscores the importance of considering sex as a variable in medical research, a consideration that could lead to more effective and personalized treatments in the future.
Looking Ahead
By highlighting the differences in how our cells respond to viral infections based on sex, this research paves the way for future studies and for the development of more nuanced and effective medical treatments. These findings promise to enrich our approach to healthcare, emphasizing the need for personalized medicine that accounts for the biological intricacies of each individual. It’s a step toward not just fighting diseases more effectively but revolutionizing the very way we approach medical treatment.